Bencherif Publishes “Oxygen-Generating Cryogels Restore T Cell Mediated Cytotoxicity in Hypoxic Tumors” in Advanced Functional Materials
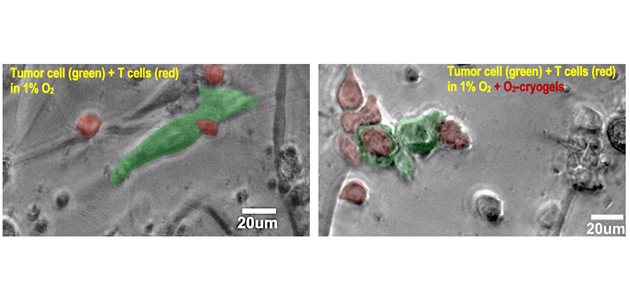
Microscopy images depicting the ability of O2-cryogels to restore OT-1 (red, T cells) cytotoxic activity and kill B16-OVA tumor cells (green) under immunosuppressive hypoxic conditions (~1% O2).
ChE Postdoctoral Research Associate Thibault Colombani (lead author), Loek Eggermont, Stephen Hatfield, Zachary Rogers, PhD’23, Mahboobeh Rezaeeyazdi, PhD’19, Adnan Memic, Michail Sitkovsky, and Assistant Professor Sidi A. Bencherif published their article Oxygen-Generating Cryogels Restore T Cell Mediated Cytotoxicity in Hypoxic Tumors in the Advanced Functional Materials journal. In this article, they report the design of injectable oxygen-generating cryogels (O2-cryogels) as a strategy to boost antitumor immune responses. These non-invasive biomaterials are used to controllably release oxygen intratumorally, reverse hypoxia-driven immunosuppression, and promote the infiltration of pre-existing tumor-fighting immune cells into solid tumors.
Solid tumors are protected from antitumor immune responses due to their hypoxic microenvironments. Weakening hypoxia-driven immunosuppression by hyperoxic breathing of 60% oxygen has shown to be effective in unleashing antitumor immune cells against solid tumors. However, efficacy of systemic oxygenation is limited against solid tumors outside of lungs and has been associated with adverse side effects. As a result, it is essential to develop targeted oxygenation alternatives to weaken tumor hypoxia as novel approaches to restore immune responses against cancer such as melanoma (skin cancer). In this article, the Bencherif lab designed syringe-injectable O2-cryogels to reverse tumor-induced hypoxia. These macroporous biomaterials were fabricated to locally deliver oxygen, inhibit the expression of hypoxia-inducible genes in hypoxic melanoma cells, and reduce the accumulation of immunosuppressive extracellular adenosine. Mechanistically, O2-cryogels enhance T cell-mediated secretion of cytotoxic proteins, restoring the killing ability of tumor-specific cytotoxic T lymphocytes (Figure), both in vitro and in vivo. O2-cryogels are a novel platform to deliver oxygen locally as a co-adjuvant in solid tumors, reinforce tumor-infiltrating T cells, and ultimately enhance tumor rejection.